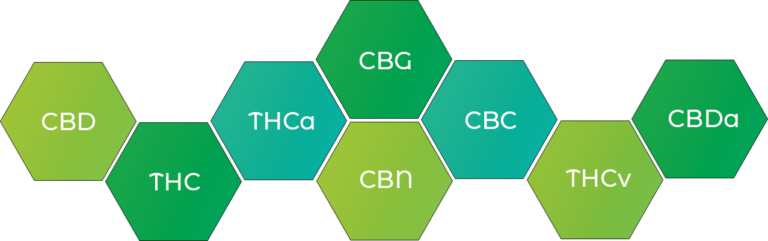
There are over 400 compounds found in the cannabis plant including what are known as ‘cannabinoids’. This group of compounds interact directly with the human body’s cannabinoid receptors. These receptors are effectively binding sites for cannabinoids and are part of what is known as the ‘endocannabinoid system’.
Two well-known cannabinoids are Tetrahydrocannabinol (THC) and Cannabidiol (CBD). CBD in particular has been gaining traction in the health and wellness market as countries begin to relax their laws around the use of this compound which clinical research has shown to have anti-anxiety and pain-relieving properties.
CBDA (Cannabidiolic Acid) is known as a carboxylic acid. This means its structure contains a carboxyl group comprising one carbon, one hydrogen, and two oxygen atoms, commonly expressed as COOH. When you expose this molecule to high temperatures, this group breaks up to form carbon dioxide and hydrogen. What’s left is CBD.
In short, CBDA is the raw form of CBD. It turns into CBD when heated. Since most extraction processes require heat, it is not easy to extract the raw CBDA compound from the plant into a concentrate for human consumption. For a long time researchers ignored CBDA because they saw it as inert: requiring decarboxylation (heating) in order to unlock its potential in the form of CBD. However, that all changed about ten years ago when a research team compared the molecular structures of CBDA with nonsteroidal anti-inflammatory drugs (NSAIDs) commonly used to treat inflammation and found their chemical structures remarkably similar. Since then CBDA has been stealing a lot of attention from its cannabis cousins largely due to its greater bioavailability: meaning the body can metabolise the compound with less effort and in less time.